Atomic-scale phenomena shape the world we experience. I explore how matter stores and transforms energy, and measure how it manifests as atomic vibrations and magnetism.

Atomic-scale phenomena shape the world we experience. I explore how matter stores and transforms energy, and measure how it manifests as atomic vibrations and magnetism.
Research


Spinning electrons create local magnetic fields that are sensitive to nanoscale perturbations. Using neutrons and microwaves, I probe how molecular vibrations disrupt these fields, ultimately limiting quantum sensing.
I study whether individual molecules can sense their local environment and how their structural oscillations interfere with that signal. My experiments search for relations between structure, movement, and information at the molecular scale.
Molecular quantum sensing


Spinning electrons create local magnetic fields that are sensitive to nanoscale perturbations. Using neutrons and microwaves, I probe how molecular vibrations disrupt these fields, ultimately limiting quantum sensing.
I study whether individual molecules can sense their local environment and how their structural oscillations interfere with that signal. My experiments search for relations between structure, movement, and information at the molecular scale.
Molecular quantum sensing
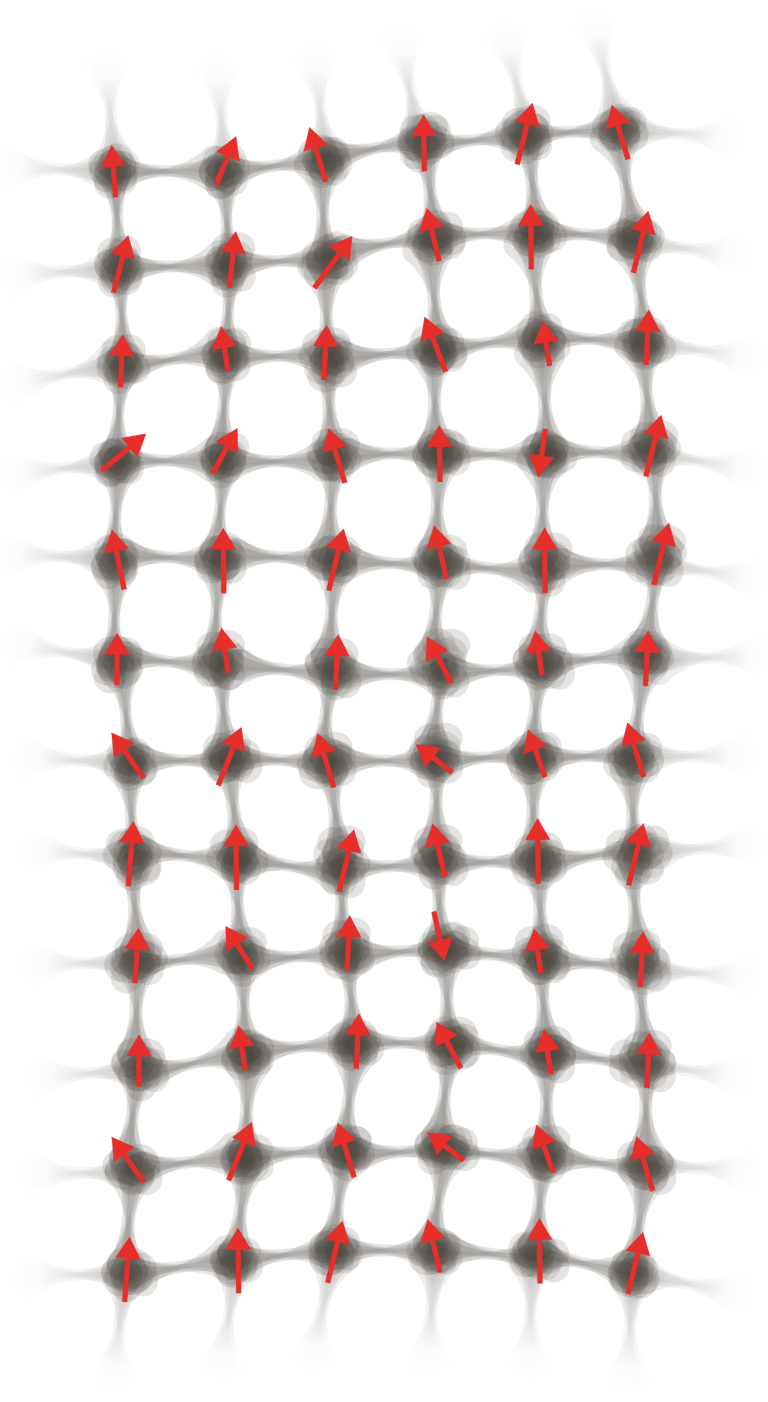
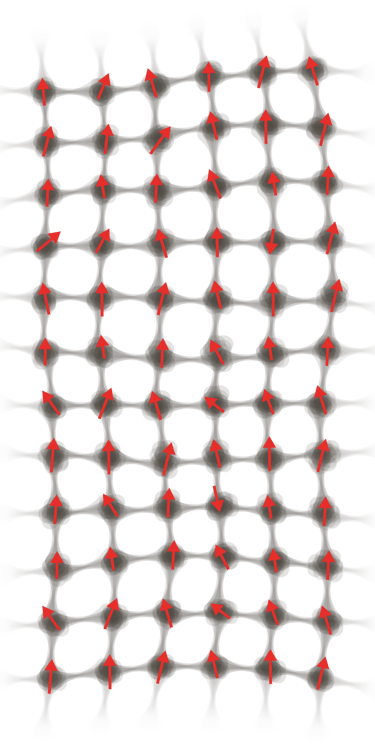
I investigate how thermal energy moves into matter and is stored within materials as atomic vibration, electronic excitation, and magnetic structure. These microscopic processes give rise to macroscopic properties such as heat capacity, stability, and phase transitions.
Atomic Pathways of Energy
Energy manifests in matter as atomic vibrations, crystalline distortions, and disruptions in magnetic alignment. Entropy statistically links these local excitations to behavior at the macroscopic scale.


I investigate how thermal energy moves into matter and is stored within materials as atomic vibration, electronic excitation, and magnetic structure. These microscopic processes give rise to macroscopic properties such as heat capacity, stability, and phase transitions.
Atomic Pathways of Energy
Energy manifests in matter as atomic vibrations, crystalline distortions, and disruptions in magnetic alignment. Entropy statistically links these local excitations to behavior at the macroscopic scale.
Energetics of Disorder
What is the role of symmetry at the atomic scale? I examine how disorder increases energy and destabilizes matter, from metallic glasses to the transition between solid and liquid.
Energetics of Disorder
What is the role of symmetry at the atomic scale? I examine how disorder increases energy and destabilizes matter, from metallic glasses to the transition between solid and liquid.
Structural Dynamics
I study how motion is shaped by structure, from the resonance of violins to the stability of large ultralight space systems. My experiments show when vibrations are absorbed and when they grow into instability.
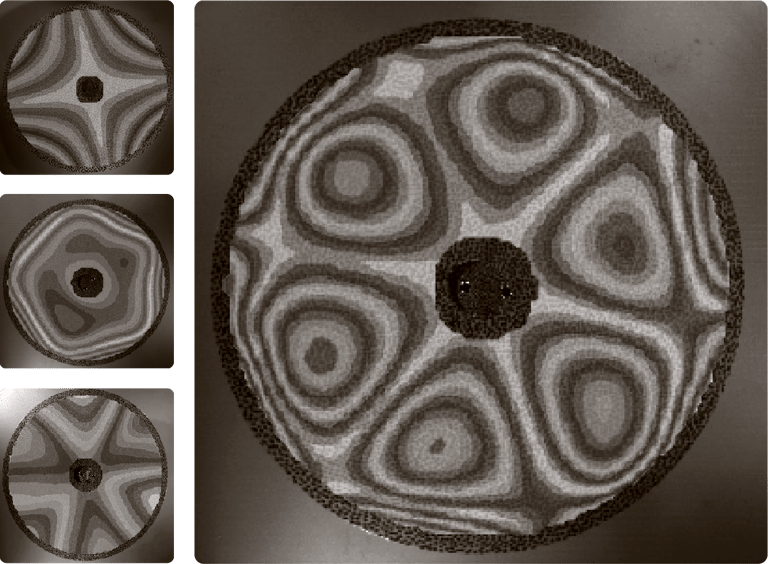
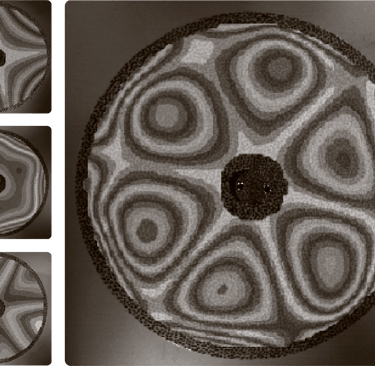
Measured vibrational modes of an ultrathin disk spinning in vacuum.
Structural Dynamics
I study how motion is shaped by structure, from the resonance of violins to the stability of large ultralight space systems. My experiments show when vibrations are absorbed and when they grow into instability.
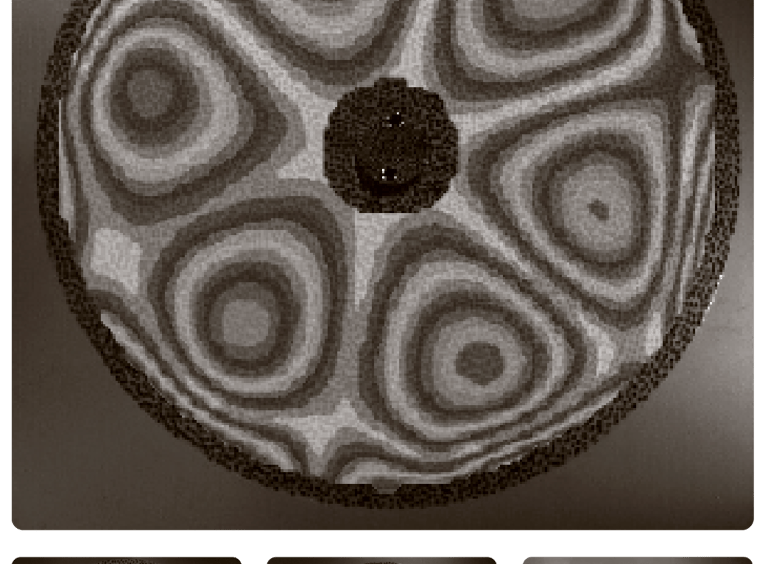

Measured vibrational modes of an ultrathin disk spinning in vacuum.




Selected News Features
Selected News Features
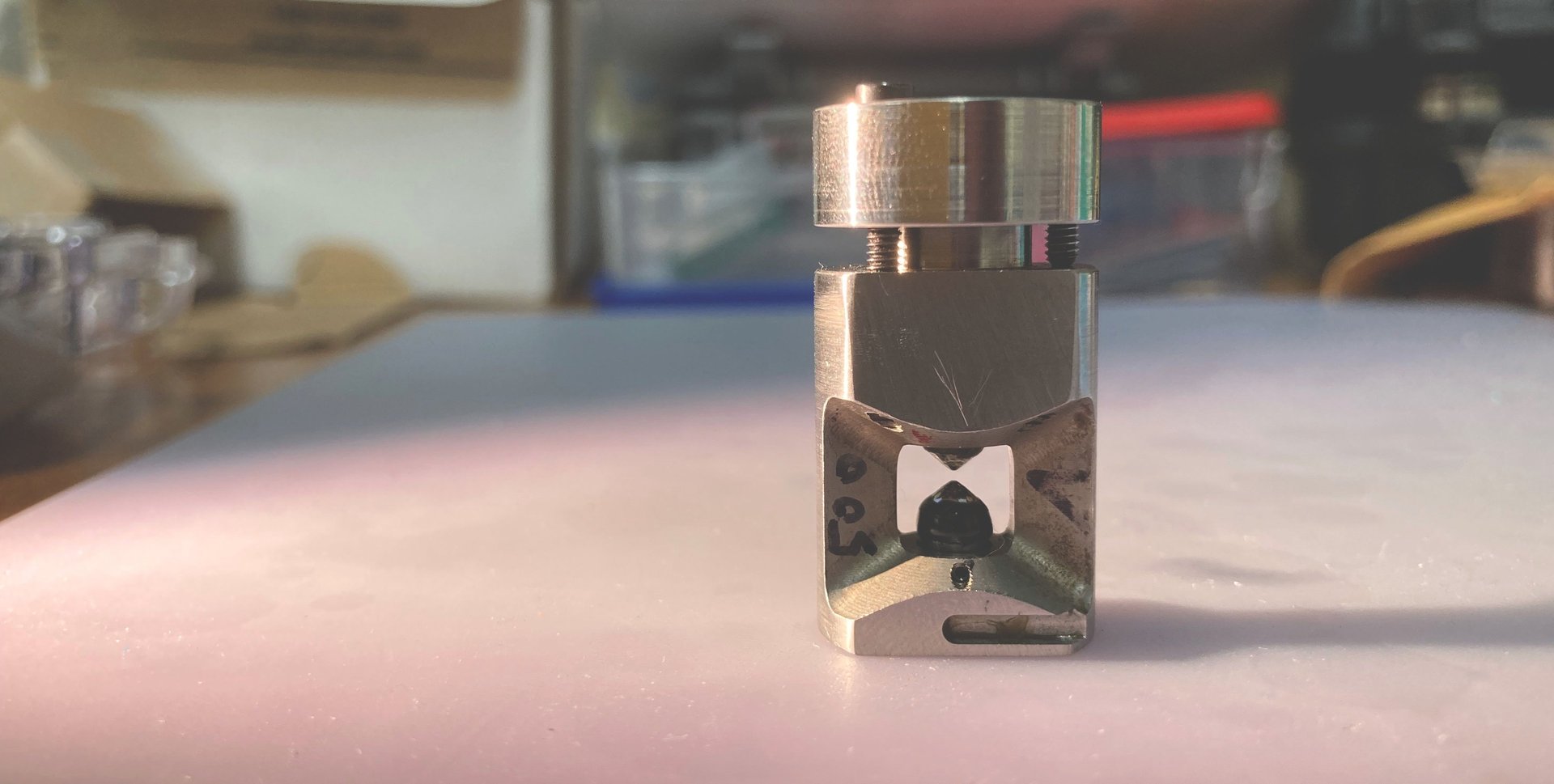

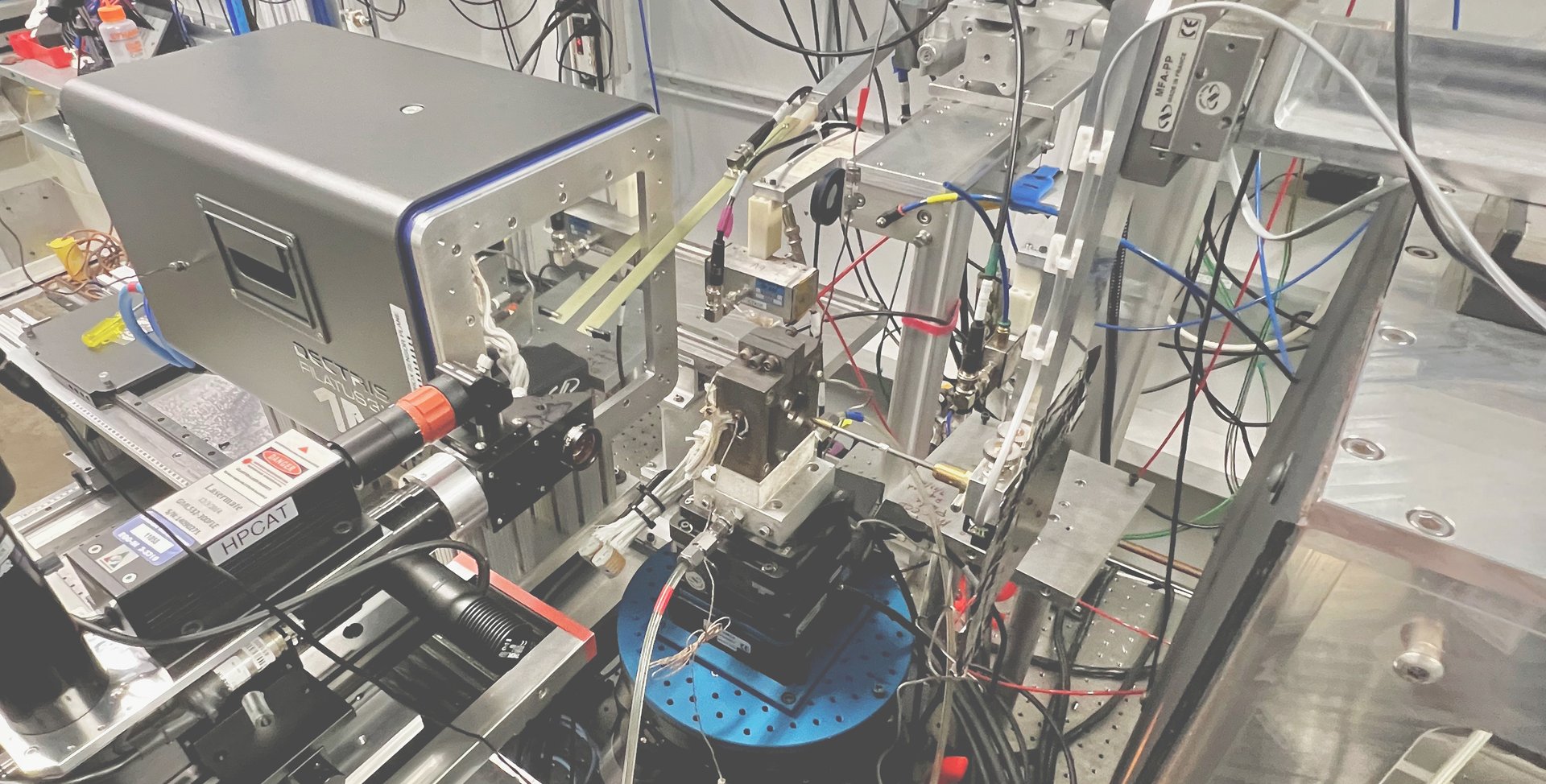
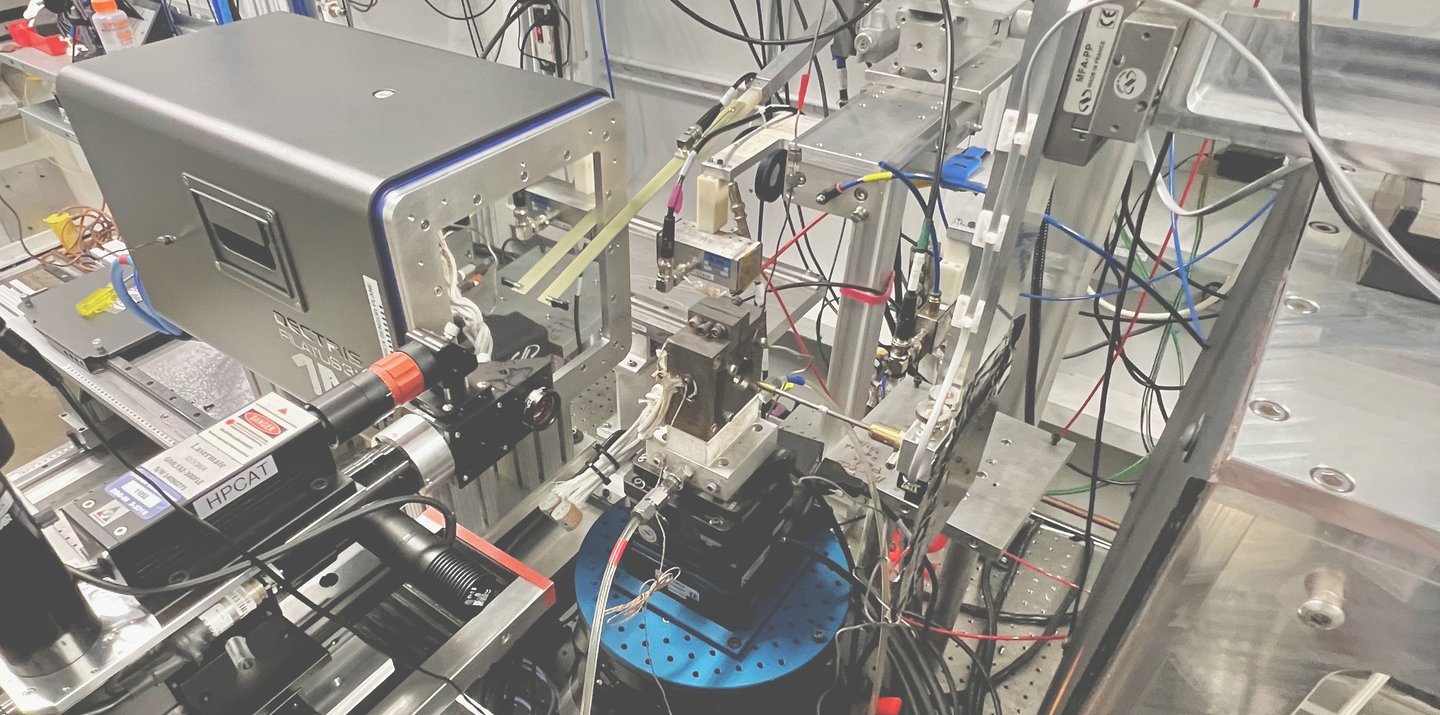
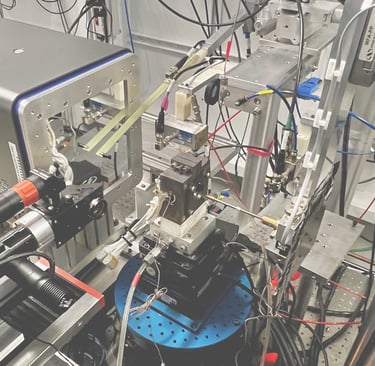
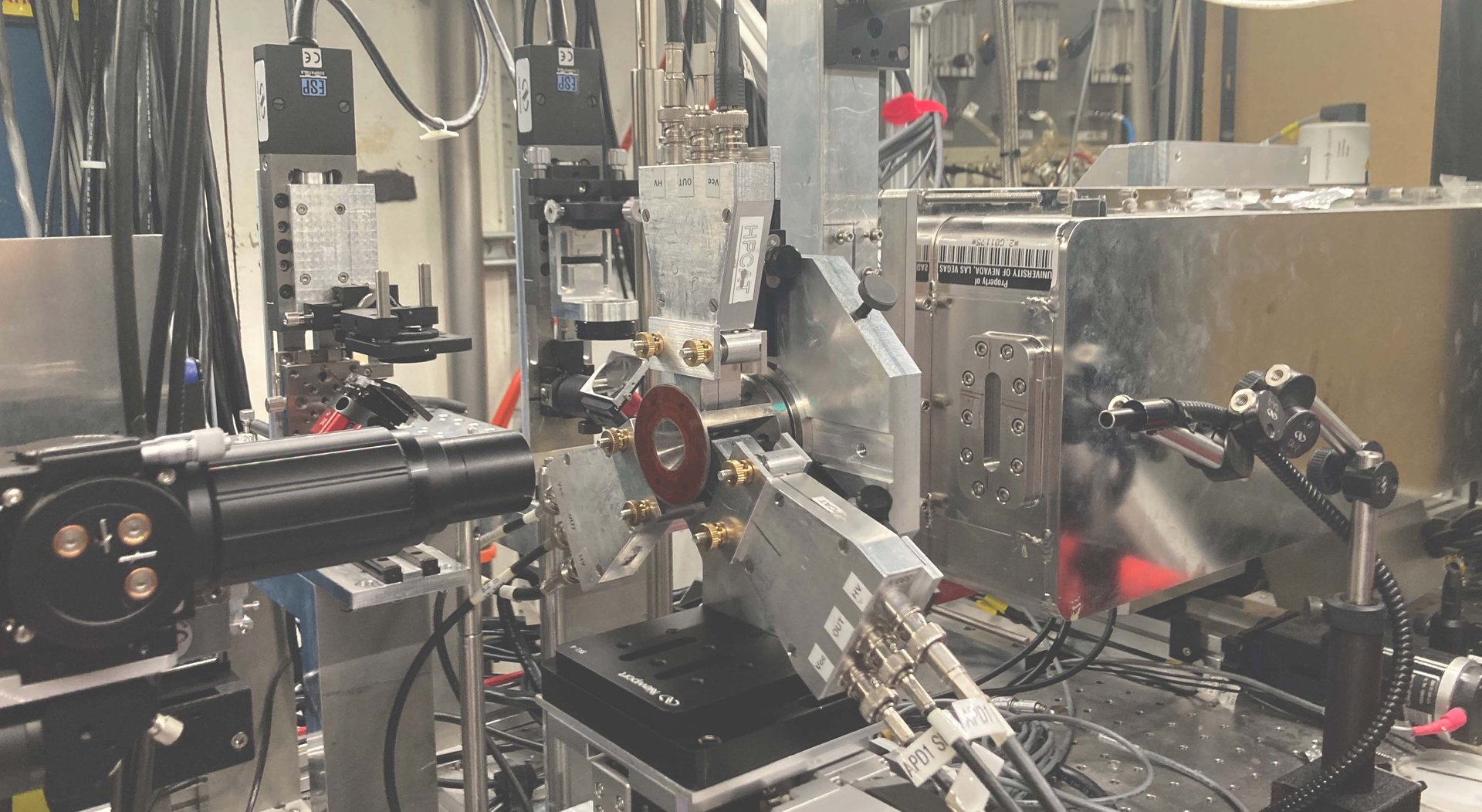
Experimental setup to measure the vibrational motion of atoms and the local magnetization of Invar materials at the Advanced Photon Source, a synchrotron at Argonne National Laboratory - IL. X-rays come out of the beryllium window (metallic box on the right), go through the sample inside the diamond-anvil cell (DAC), and scatter into three detectors placed closely around the DAC.












